Cell & Gene Therapies
Cell and Gene Therapies (CGTs) are slowly transitioning from a moonshot idea to a mainstream (albeit complex) therapy. Dozens of CGT programs have now secured FDA approval. Hundreds more are in clinical trials, generating positive data across an expanding list of diseases.
The path to success hasn’t been easy. Due to unique development needs, CGTs require adaptations to everything from the biopharma supply chain to how FDA drugs are reviewed and approved.
Scroll down to learn more about these era-defining therapies and how they’re changing the medical landscape.
The Science Behind
Cell and Gene Therapies
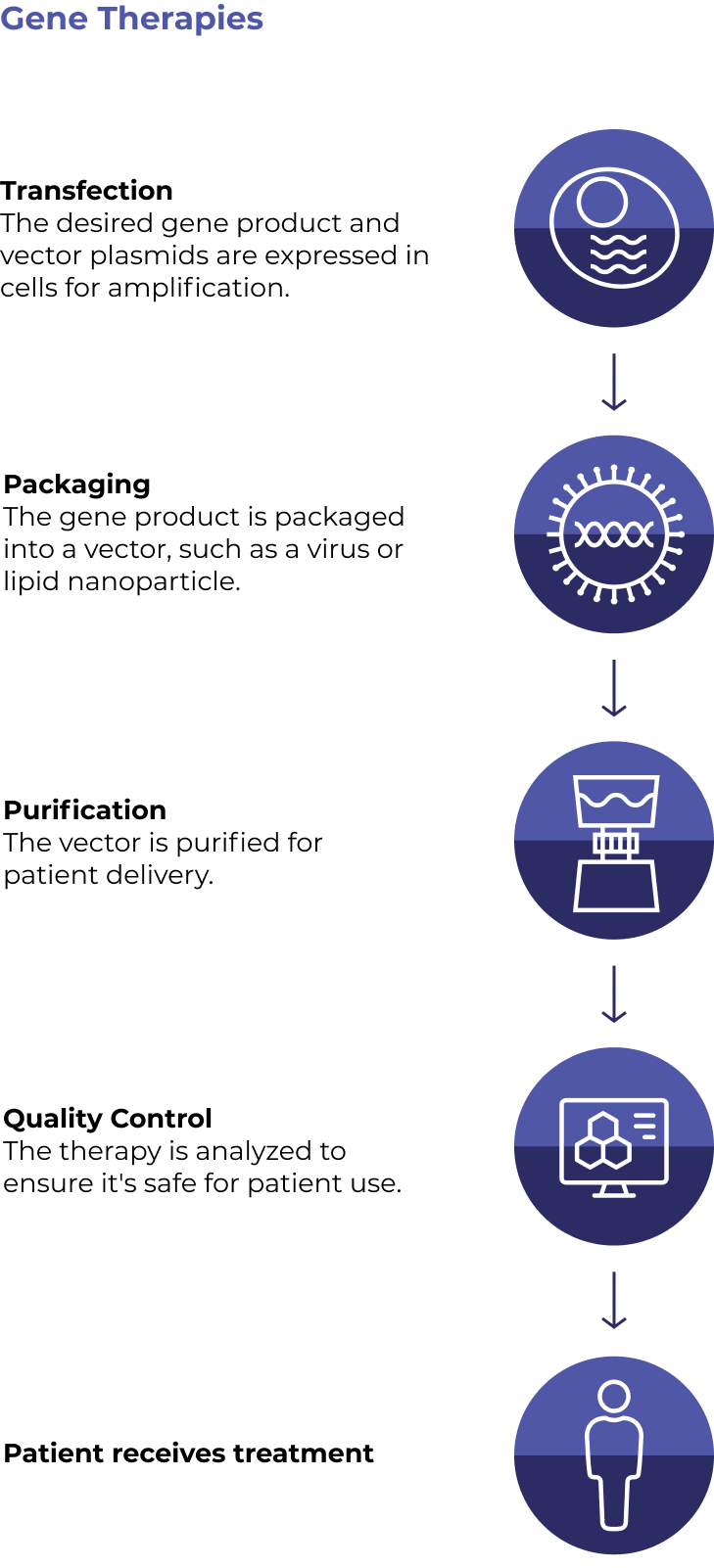
Gene therapies work by silencing a dysfunctional gene or introducing a new beneficial gene into target cells. A related approach – gene editing – uses Nobel-prize winning technology to fix the faulty gene directly.
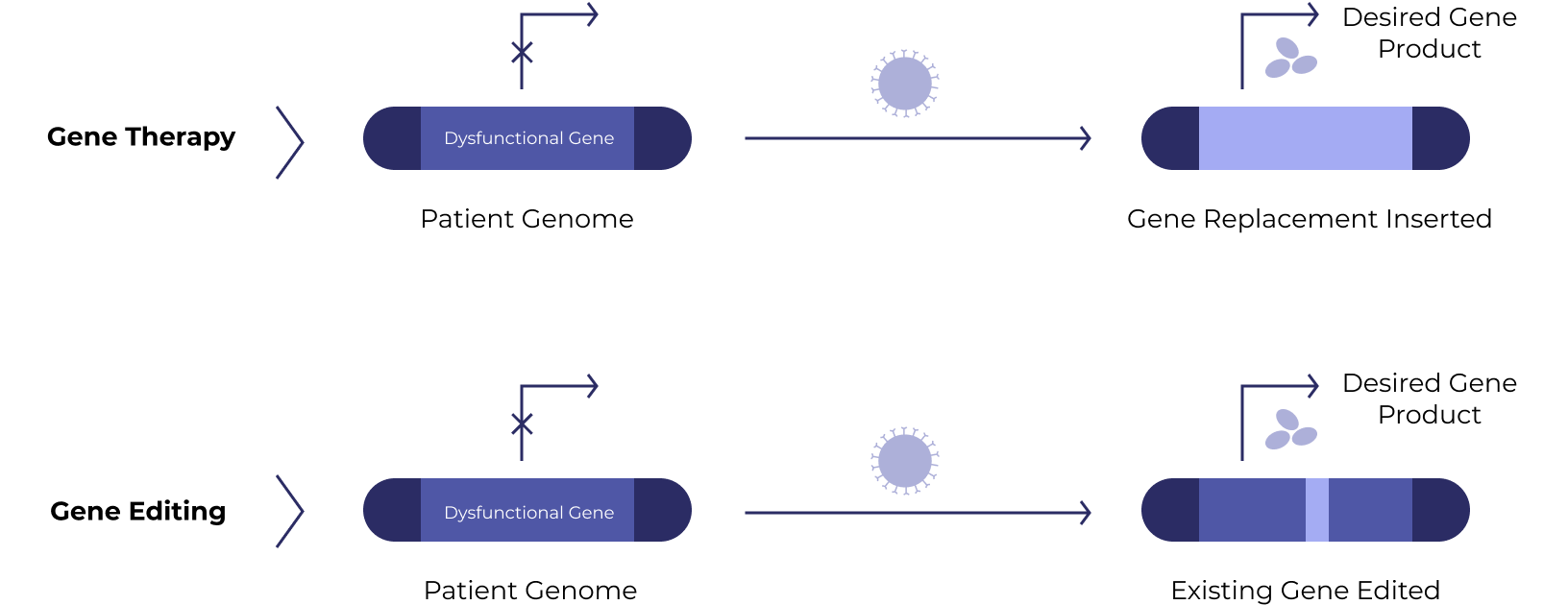

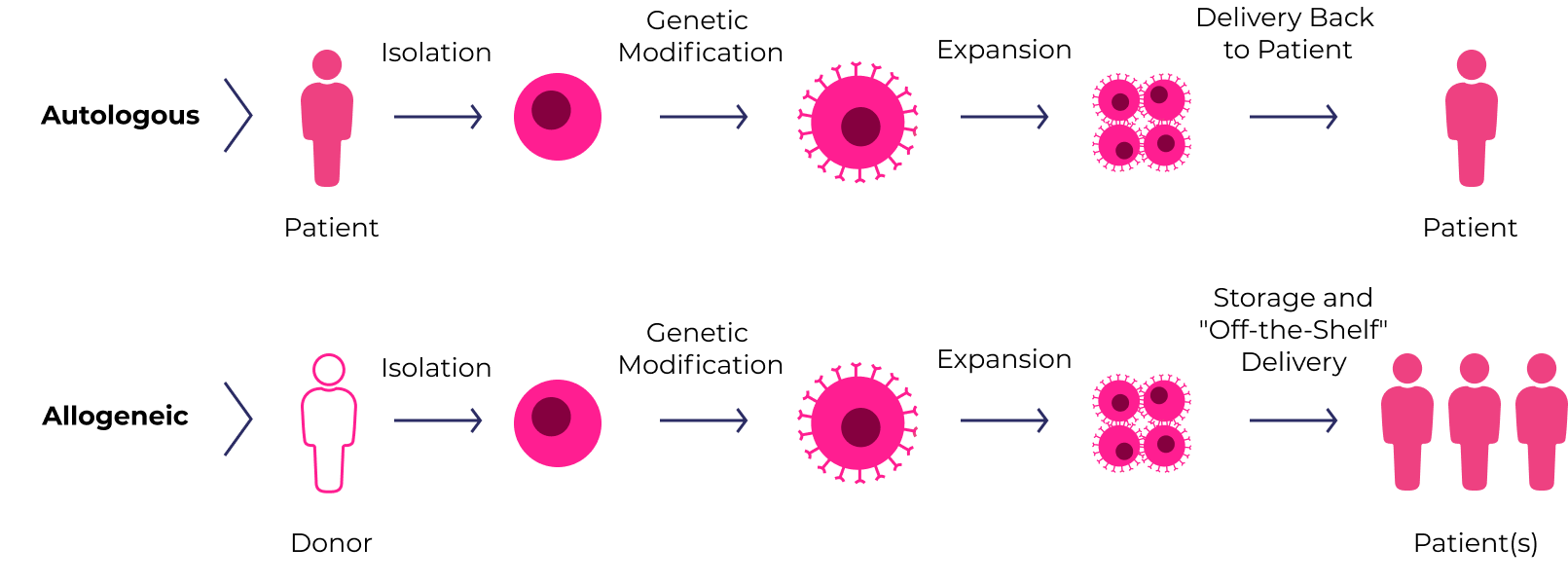
Cell therapies use human cells collected from the patient (autologous) or a healthy donor (allogeneic). The cells are expanded and often genetically engineered to enhance their disease-fighting potential.

Early Applications
Gene Therapies
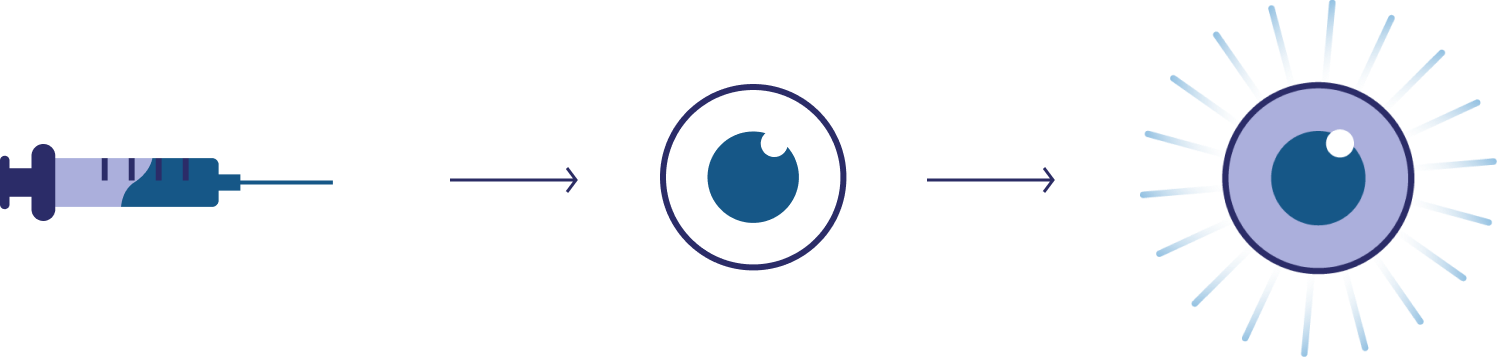
Luxturna®, one of the first FDA-approved gene therapies, uses an adeno-associated virus (AAV) to transport healthy copies of a key gene (RPE65) into retinal cells. This improves vision in patients with RPE65-associated blindness.

Cell Therapies
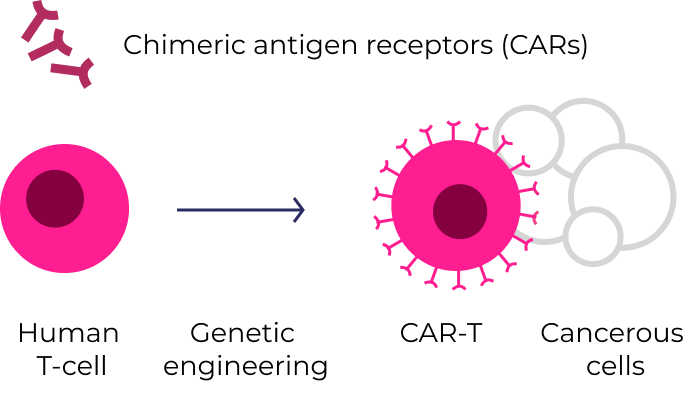
CAR-T therapies (e.g., Yescarta® , Abecama®, and Kymriah®) are created by genetically engineering a patient’s own T-cells. Once reinfused, they seek out and destroy cancer cells that express the target protein.

That day back in 2010 when I was infused with my CAR-Ts, and my tumor cells disappeared – it meant there was a whole new treatment paradigm.
— Doug Olsen, the second CAR-T patient (STAT News)
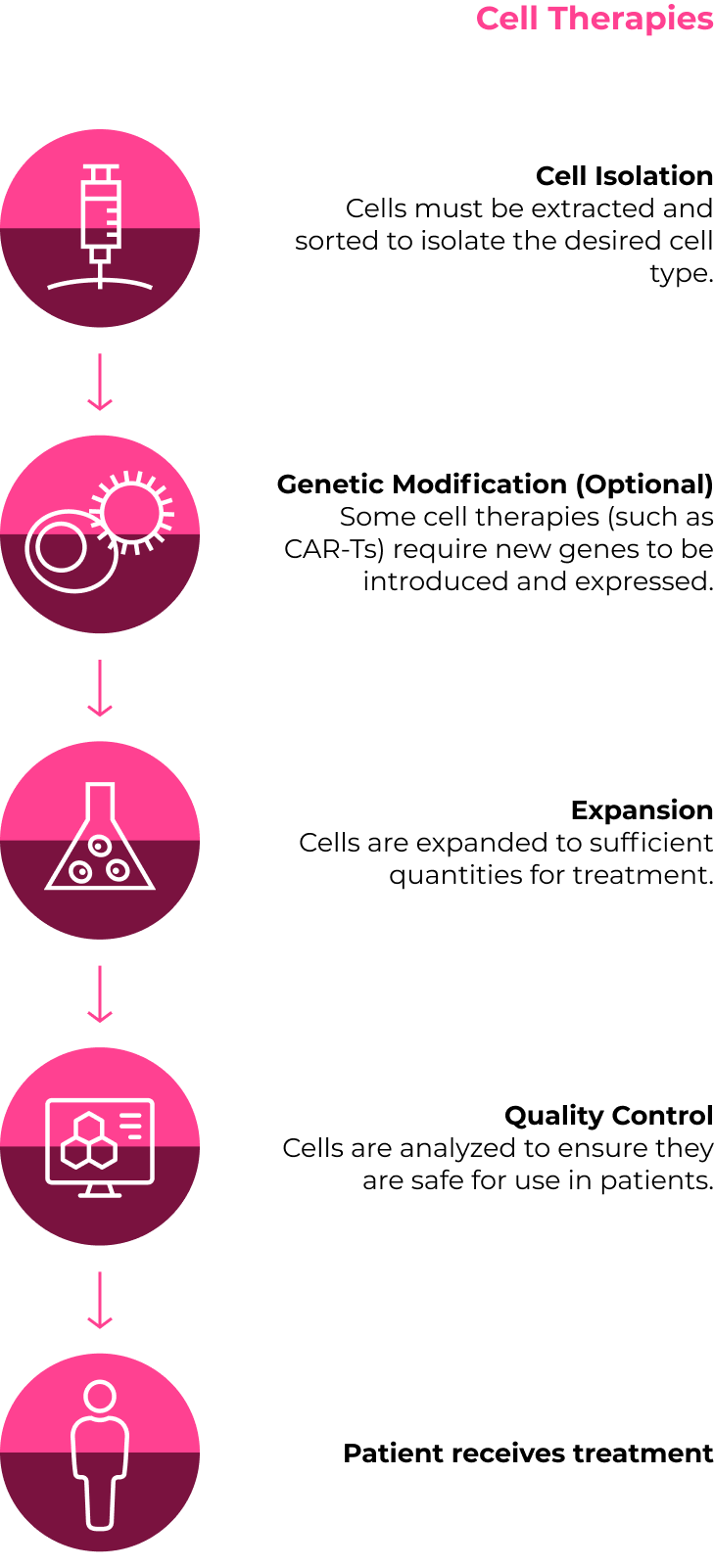
The CGT Treatment Process
While there are many different approaches, below are two sample individualized treatment plans


Fantastic progress has been made across all aspects of CGT development and manufacturing. However, some core challenges still need to be addressed to enable broader patient access. Here are some of the areas that innovators are working hard to improve so that the full potential of CGTs can be realized.


Production Speed
Experts are looking for ways to streamline the highly individualized and complex production of CGTs to provide therapies in a timely manner.


Consistency
For patient safety and regulatory approvals, CGTs require stringent analytical testing to create consistency across therapies and to standardize every batch.

Scalability
Industry experts are exploring news tools and techniques to rapidly scale up or scale out CGT production. This is crucial for lowering costs and increasing patient access.
Cell & Gene Therapy By The Numbers


As of February 2024, the U.S. FDA’s Office of Tissues and Advanced Therapies (OTAT) had approved 35 cell and gene therapies.1


As of 2024, >2,500 companies were developing cell and gene therapies. Nearly 1,800 therapies were in clinical trials.2


Annual investment in CGTs is projected to reach $17.3B in 2024, doubling to almost $40B by 2028.3
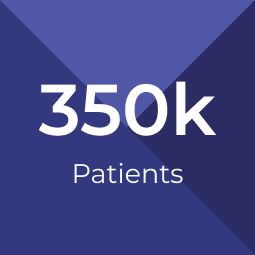

By 2030, an estimated 350,000 patients will have received a cell or gene therapy, with between 30-60 unique products approved.4
References:
- Approved Cellular and Gene Therapy Products | FDA. Accessed November 9, 2023. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products
- Cell and Gene Therapy Sector Data – Alliance for Regenerative Medicine. Accessed April 11, 2024. https://alliancerm.org/data/
- Cell and Gene Therapy Market Analysis, Precise Outlook And Trends Report To 2033. Accessed April 11, 2024. https://thebusinessresearchcompany.com/report/cell-and-genes-therapy-global-market-report
- Quinn C, Young C, Thomas J, Trusheim M, MIT NEWDIGS FoCUS Writing Group. Estimating the clinical pipeline of cell and gene therapies and their potential economic impact on the US healthcare system. Value Health. 2019;22(6):621-626. doi:10.1016/j.jval.2019.03.014

What’s your story?
CG Life has a roadmap to help construct your narratives and engage audiences across the clinical pipeline. Let’s bring your cell or gene therapy to those who need it.